Programs & Research
Explore our current research and clinical trials involving the QTORIN™ platform.
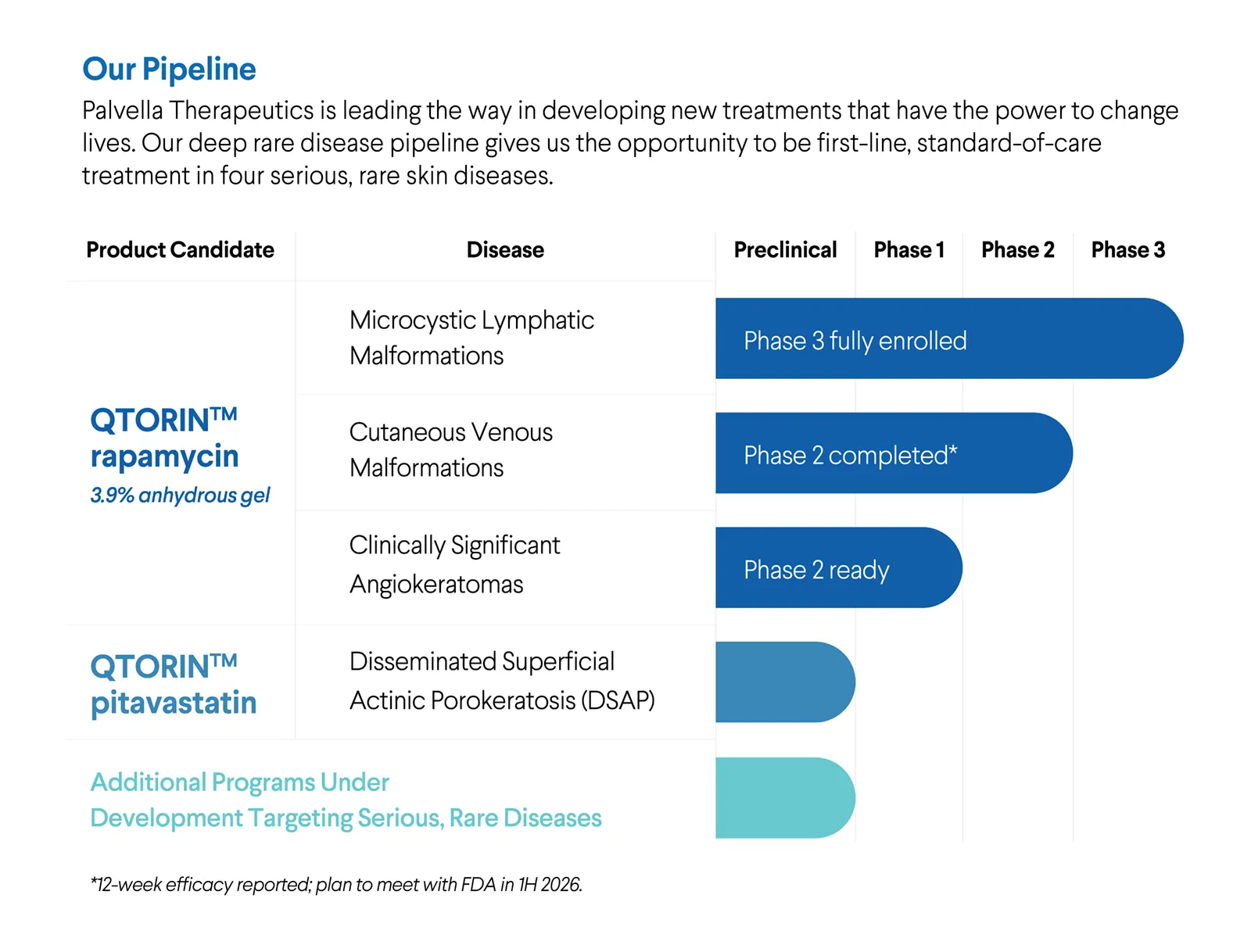
Our Pipeline
Palvella Therapeutics is leading the way in developing new treatments that have the power to change lives for those with rare skin diseases.
The Power of QTORIN™ rapamycin
Rapamycin has long been explored as a potential treatment for skin diseases, but topical treatment presents many challenges including high molecular weight, chemical and physical instability and excessive systemic absorption through skin penetration.
QTORIN rapamycin has been developed to overcome these challenges. QTORIN provides targeted rapamycin delivery to the main area of concern, usually within the deep layers of the epidermis or dermis.1

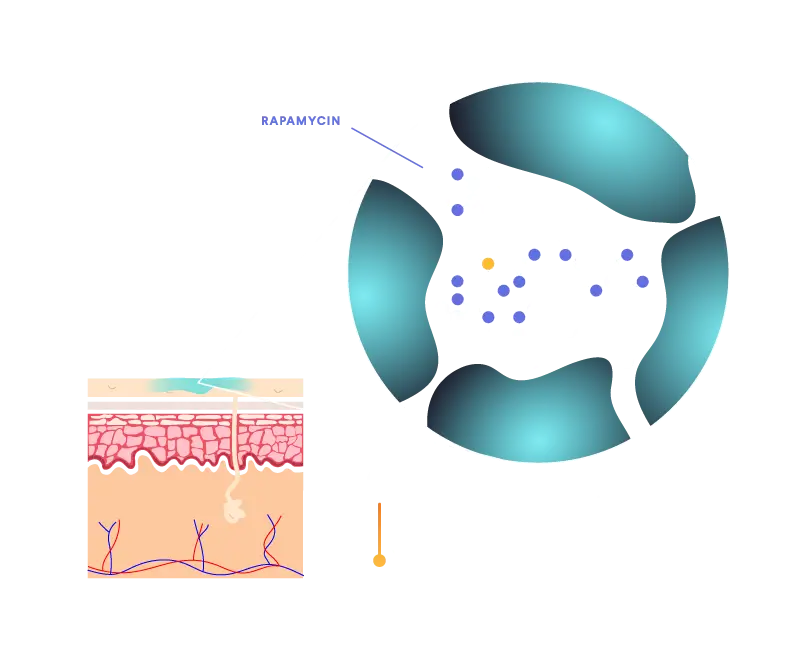
QTORIN™ helps stabilize rapamycin, which is highly susceptible to degradation. Our QTORIN™ rapamycin 3.9% is stable at room temperature in our pump dispenser for extended periods.
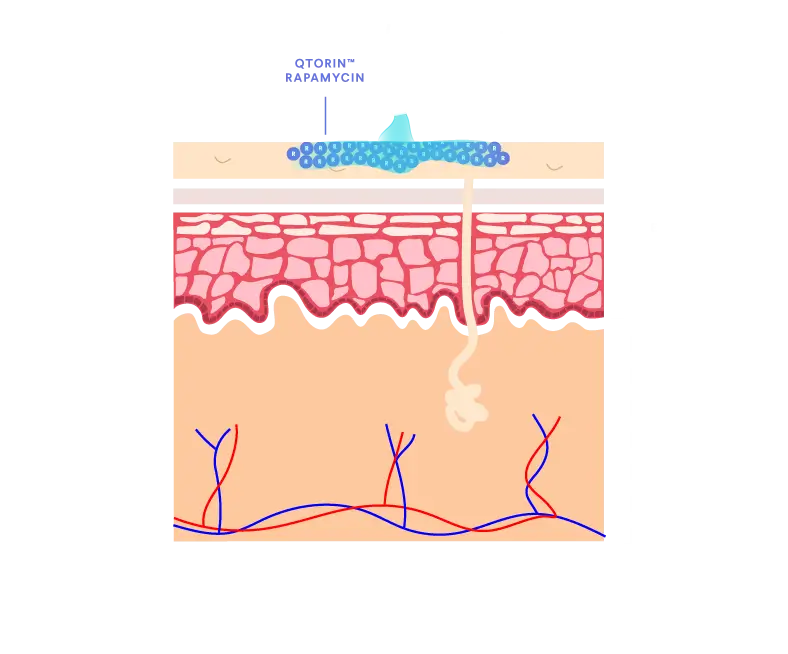
QTORIN™ rapamycin 3.9% topical gel is a high-strength formulation that provides local and target therapeutic effect with minimal systemic absorption.1
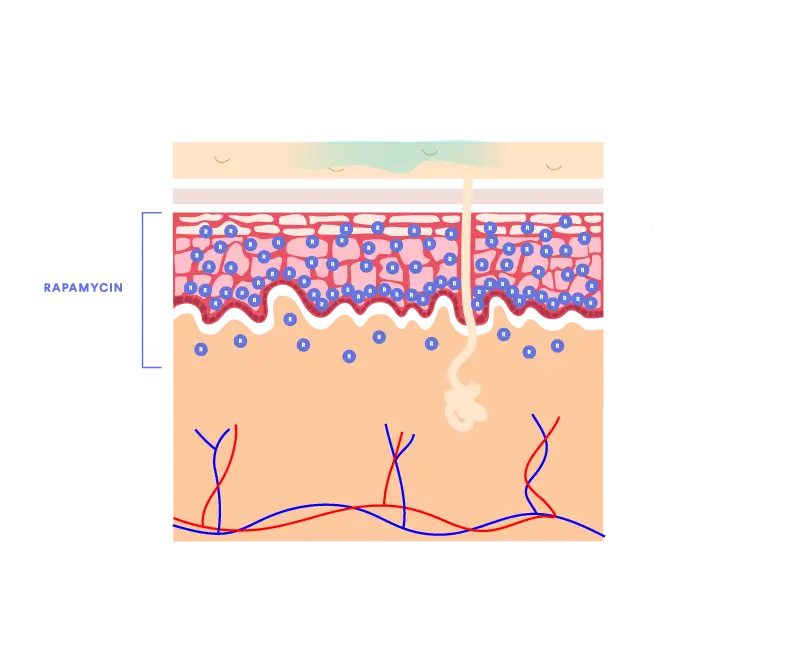
Prior to the QTORIN™ platform, rapamycin exhibited very poor skin absorption due to its high molecular weight and poor stability. QTORIN™ rapamycin 3.9% formulation yields optimal epidermal and dermal distribution with systemic absorption achieving only picogram levels in clinical studies.1

Microcystic Lymphatic Malformations
Microcystic Lymphatic Malformations (Micro LM) is a rare genetic disease of the lymphatic system that presents on the skin, characterized by abnormal vessels or cysts that lead to chronic lymphorrhea (abnormal flow of lymph fluid), bleeding, and a risk of severe life-threatening infections.
While most individuals are diagnosed early in life, the disease can become more pronounced as a person ages and goes through puberty, and there are no FDA-approved therapies.
Targeting Micro LM
QTORIN™ rapamycin 3.9% is a novel targeted topical therapy being studied Micro LM. QTORIN™ rapamycin 3.9% is designed to target the PI3K/mTOR pathway, which is overactivated in Micro LM. QTORIN™ rapamycin 3.9% topical gel delivers rapamycin deep into the dermis, the area where the disease originates.
Palvella has successfully completed a phase 2 clinical study for people with this disease and has been awarded FDA Breakthrough Therapy Designation, FDA Orphan Drug, and Fast Track Designation for this program.

Cutaneous Venous Malformations
Cutaneous venous malformations are the result of an error in vascular embryogenesis resulting in the formation of thin-walled, dilated venous channels with sparse, clumped smooth muscle cells.
Cutaneous venous malformations generally present as localized malformations with variations in age of presentation, lesion size, and location. There are no FDA-approved treatments indicated for cutaneous venous malformations.
Other mTOR-Driven Skin Diseases
Palvella is continuing research into other mTOR-driven diseases, building on our clinical experience with QTORIN™ rapamycin. Subscribe to our email list to get updates on future clinical trials.
References
1. Data on File