Nasdaq, Inc./ Vanja Savic
Rare Is Everywhere.
Let’s Treat It.
Building the leading therapeutics company focused on rare genetic skin diseases with no approved therapies.
Palvella (pɑlʋelːɑ) : a Finnish word meaning “serve”
: a Finnish word meaning “serve”
Palvella Therapeutics exists to address one of the most urgent gaps in medicine: rare skin diseases with no approved therapies. We are driven by a mission to serve patients who have been left without options, focusing our efforts where the unmet need is greatest. Everything we do is anchored in advancing treatments that can meaningfully change lives where no solutions currently exist.
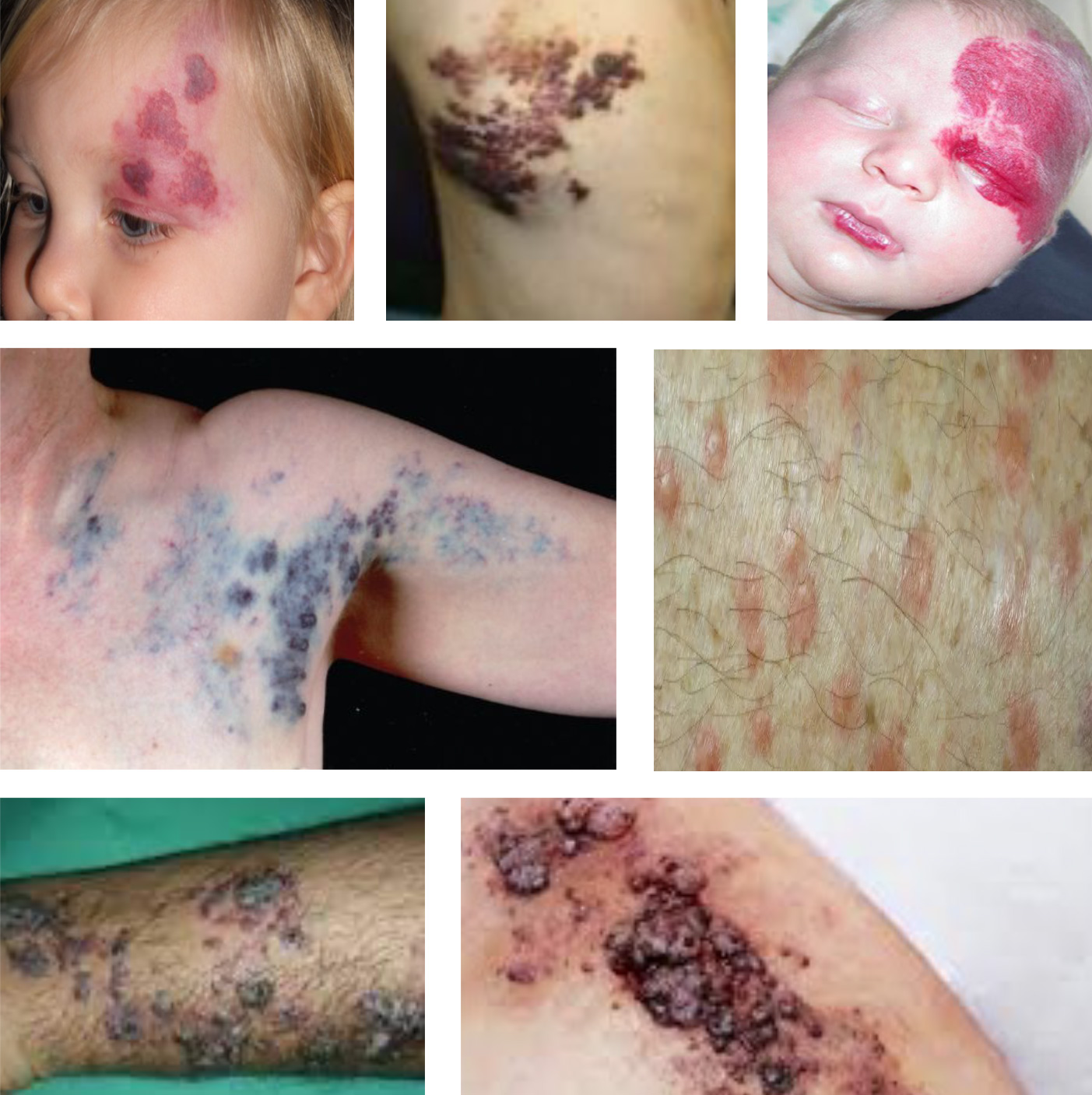
A Visible Need
1 in 10 Americans have a rare disease.1
Genetic skin diseases are visible, often debilitating, and most lack even a single FDA-approved therapy. Among nearly 600 rare dermatologic diseases, less than 2 percent currently have an FDA-approved therapy.
Our Platform:
QTORIN™
Developing targeted topical treatments for certain rare skin diseases is hindered by technical challenges including achieving therapeutic drug concentrations and chemical stability while minimizing system absorption of the drug—issues we’re addressing with Palvella’s QTORIN™ platform.
Since its invention in 2017, the QTORIN™ platform has undergone several years of research and investment, emerging as a new, revolutionary drug delivery platform. Applied topically rather than taken orally, QTORIN™ has the power to transform the current treatment landscape—and transform lives in the process.
Our Programs
QTORIN rapamycin, Palvella’s lead product candidate, is a novel, patented 3.9% rapamycin anhydrous gel, which aims to harness the potential therapeutic benefits of rapamycin, a mammalian target of rapamycin (mTOR) inhibitor.
QTORIN rapamycin is currently in development for the treatment of microcystic lymphatic malformations (Phase 3 SELVA study), cutaneous venous malformations (Phase 2 study), and other serious, functionally debilitating skin diseases driven by the overactivation of the mTOR pathway.


Corporate Profile
Palvella Therapeutics is a biotechnology company specializing in developing and commercializing medicines for patients with rare dermatological diseases. Headquartered in Wayne, Pennsylvania, Palvella’s leadership and scientific management teams have a proven track record of success and a strong commitment to leading the way in the advancement of targeted treatments for rare genodermatoses.
References
1. “1 in 10 Americans have a rare disease” to Rare Disease Facts.” Global Genes, 27 Sept. 2022, Source.